Structures
This section talks about the importance of structures in organic chemistry and how it compares to most basic compounds that you will have seen until this point. Here we will cover:
- The limitations of the basic molecular formula
- How we can show what a compound looks like
- The different ways we can represent this structure so we can communicate it to others
Basic molecular drawings
Up until now we are familiar with seeing structures written as a molecular formula. One particularly familiar structure is water; which we write as H2O. From this molecular formula, and with a little knowledge of basic chemistry, we can work out what the structure of water looks like. And here it is.
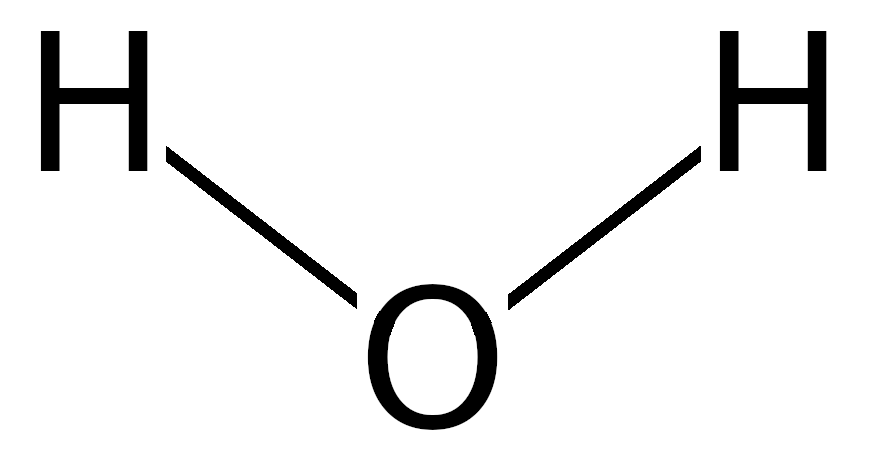
In this form we have kept the elemental symbols to represent the different atoms, and the lines represent the bonds between the atoms.
A single solid line represents a single covalent bond where only 2 electrons are shared. Two solid lines side-by-side show a double bond where 4 electrons are shared, and lastly three solid lines together represent a triple bondm where a total of 6 electrons are shared between the atoms.
This way of writing the structure also shows you the approximate arrangement of the molecule and the “bent” shape.
This form is useful but it still has limitations because this is still only a 2D version.
To get a more accurate representation we can draw the molecule in 3D (or use a modelling kit) in two ways. One is called a “space-filled” model. In this model the spheres represent the volume taken up by each atom; the nucleus all the way to the outer electrons.
We must remain aware that this whole volume is not solid (even though it looks it in this model). In fact most of that volume is the electron cloud - the large space where the tiny electrons are found.
This form is very good for understanding the size of the individual atoms and how they fit next to each other, and it also shows the lack of space when considering reactions with other molecules.<\p>
The biggest problem with this form is that it is not possible to see the type of bonds between atoms. The other common 3D model of a molecule is the “ball and stick”.
Here is a comparison of the two models side by side:
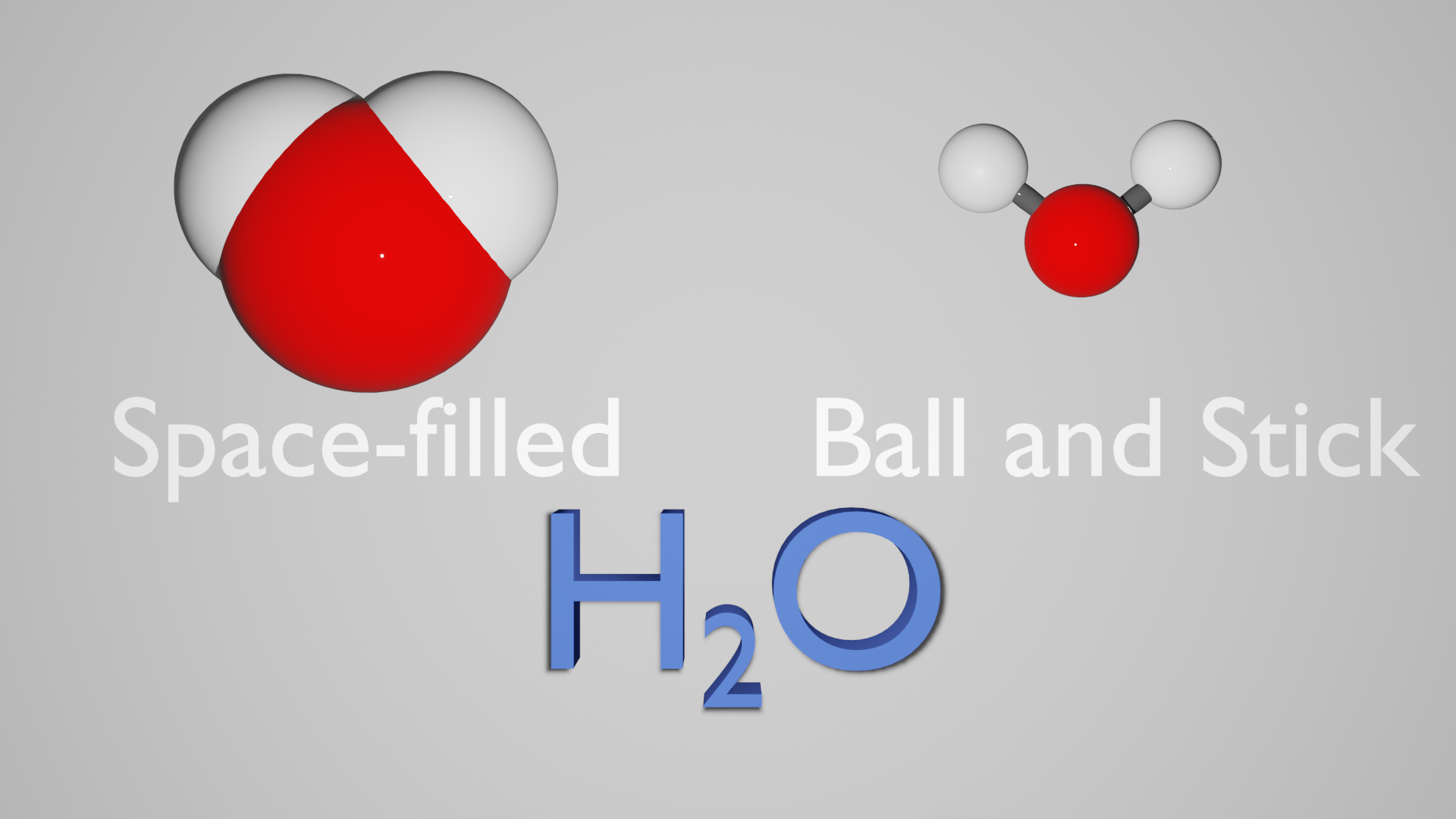
The balls in this model represent the atoms, and the sticks are the bonds. In this form, the size of the balls do not usually change, therefore we get no idea of the relative sizes but we can now clearly see the type of bonds between the atoms. All of these models, though different, represent a molecule of water.
We will now go back to the molecular formula. In this basic form, it is sufficient for basic compounds. For example, all of these structures can easily be worked out from their molecular formulae, and there is no chance of confusion.
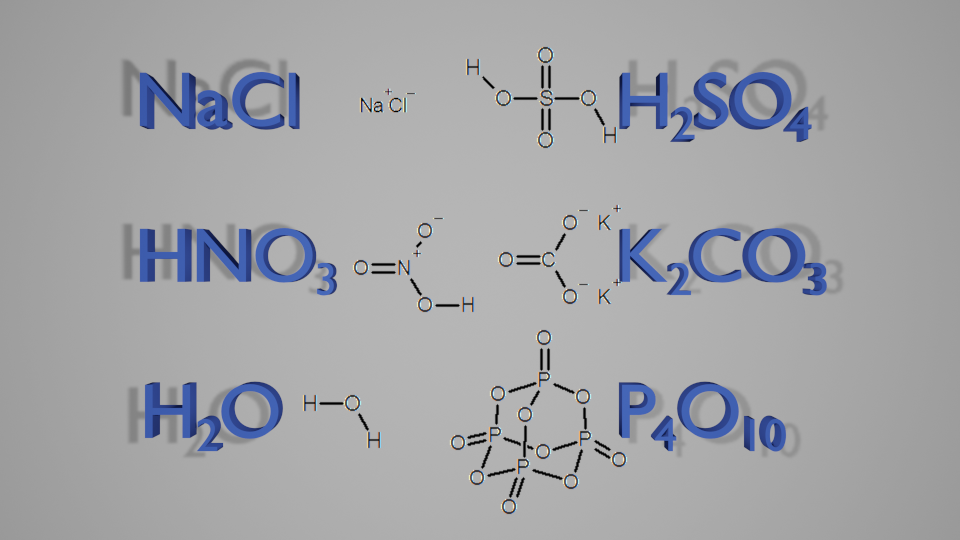
But what about something like this?
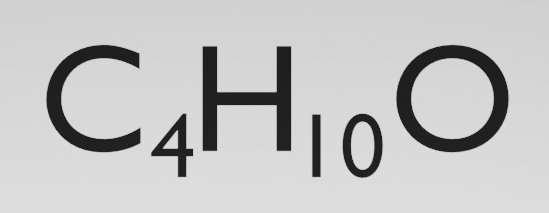
The molecular formula tells us that we have 4 carbon atoms, 10 hydrogen atoms and 1 oxygen atom. How does this molecular formula relate to the actual structure of the molecule?
Well, as you can see, it doesn't. There are 7 possible ways we can arrange these 10 atoms and we have no idea which one we are talking about:
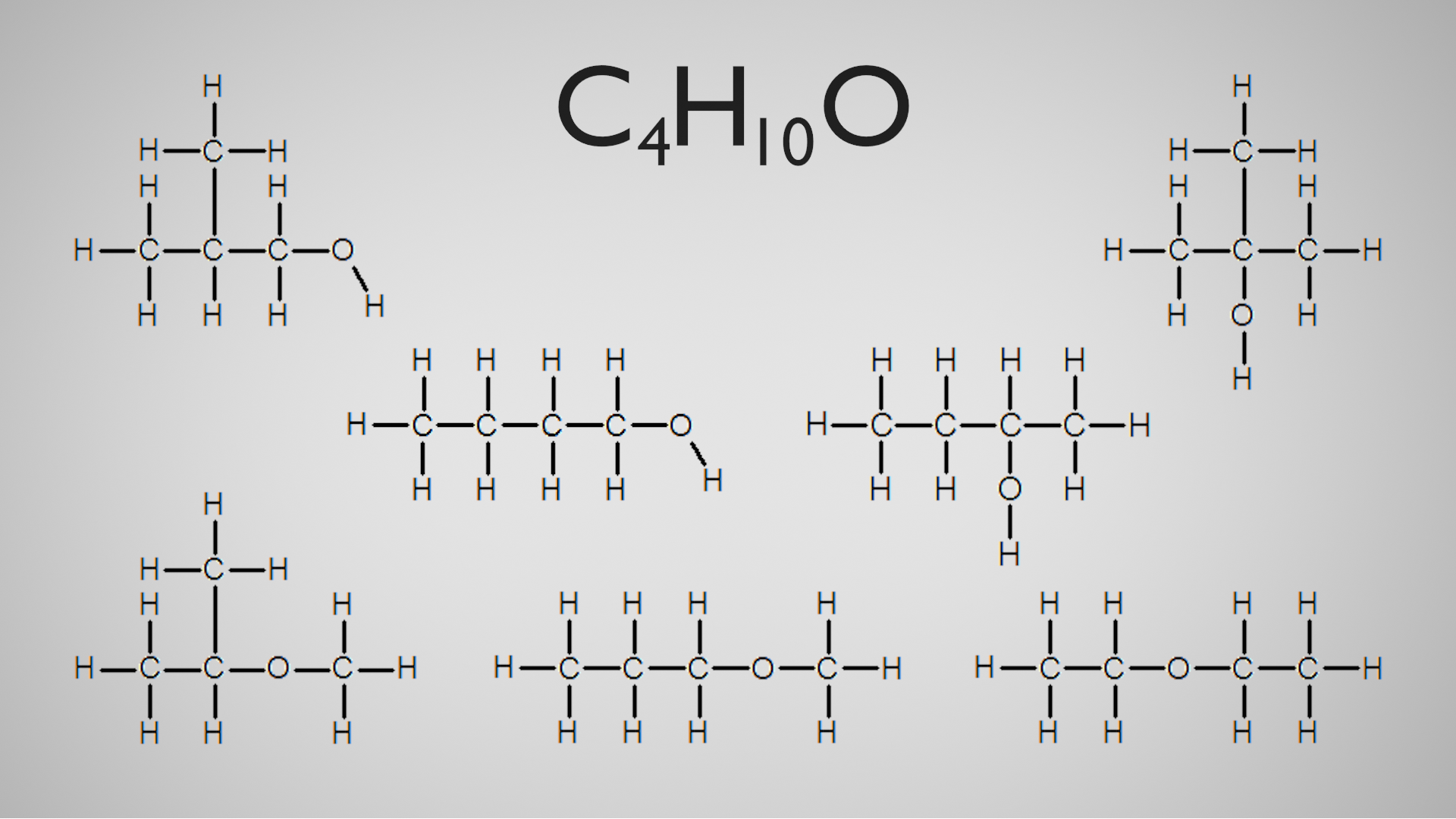
All of these arrangements are showing different compounds, with some very different properties. Not only would it be bad chemistry to not specify which of these molecules we are talking about, but it can also be potentially very dangerous. We can see from these examples, that the basic molecular formula is not adequate for these molecules. How do we get around this?
In organic chemistry we can draw the structures out, or use an extended form of the basic molecular formula, that describes the structure. First of all, here are the same structures written in the extended molecular formula:
We can see here that the side chains are represented in brackets and certain features of the molecule such as the O in the middle are used as separation points.
When it comes to drawing the structures out, this is very useful in chemistry, especially when we are examining reactions and how they occur. There are several different common ways of drawing chemical structures.
We can draw in all the atoms and the bonds. To make it more representative of the shape of the molecule we can even ensure the shape is crudely correct, like this: If we are drawing a lot of molecules, we might want to make it easier to draw. One thing we can do is to omit some of the atoms. The first to go are the non-essential hydrogen atoms.We can do this because each neutral carbon atom will have 4 bonds to other atoms. These bonds can be in the form of 4 single bonds, 1 double bond and 2 single bonds, 2 double bonds, or 1 triple bond and 1 single bond. This means that if the hydrogens are omitted then we can count the number of bonds which are shown around the carbon atom, and fill in all the missing bonds with a single bonded hydrogen atom.
We can go one step further when using a zigzag shape. In this form we can even remove the carbon atoms. Instead each “node” is then showing a single carbon atom. The hydrogen atoms can then be filled in as before.