Introduction to Reactions
Reactions
This section will study "chemical reactions" in more detail.
By the end of this section we will have covered the following:
- What is a reaction?
- What do we need for a reaction to occur?
- Factors affecting a chemical reaction
So the first point to address is:
What is a chemical reaction?
Essentially, a chemical reaction is the conversion of one or more substances (which we call reactants) into one or more different substances (called products). It is important to realise that the amount of atoms in the reactants must be exactly equal to the the number of atoms in the products. As the chemist Antoine Lavoisier stated in what became The Law of Conservation of Mass:
Matter is neither created nor destroyed, it is passed from one form to another.*
You may already be familiar with the use of a chemical equation, such as this one:
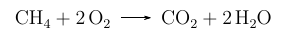
A chemical equation is pretty much the same as any mathematical equation but here we show the formulae of the reactants that we start with and the formulae of the products which are formed and we use an arrow rather than an equals sign. Notice that the number of each atoms on one side of the arrow is exactly the same as the number of each of the atoms on the other side.
* This is only applicable in a closed system (meaning the substances cannot escape).